Recently, Professor Yuan Kexin’s team from the School of Biomedical Engineering at Tsinghua University published a research paper in Cell titled “VIVIT: Resolving trans-scale volumetric biological architectures via ionic glassy tissue.” This work introduces and validates an innovative tissue-processing method, VIVIT, which enables high-fidelity three-dimensional imaging of biological tissues under a glassy state. TP MedTech contributed to the study as a co-author institution.
VIVIT overcomes three long-standing bottlenecks in tissue clearing: the trade-off between transparency and structural integrity, fluorescence signal loss, and incompatibility with freeze–thaw preservation and sectioning. This breakthrough opens new possibilities for applications in basic research, drug development, and AI-assisted diagnostics.

Three-dimensional tissue structures hold rich biological information and are key to understanding how living systems function and how diseases develop. But for decades, researchers had to rely mainly on two-dimensional slices. Samples were cut into dozens or even hundreds of layers and then pieced back together. The process was slow and labor-intensive, and physical cutting often led to deformation or breaks in the tissue, causing gaps in spatial information and even misinterpretation.
To address these challenges, tissue-clearing technologies have gradually emerged. By using chemical methods to render intact tissues optically transparent, researchers can perform 3D imaging without physical sectioning. However, most existing approaches still face limitations. Issues such as tissue swelling or shrinkage, fluorescence signal loss, and fragility during freezing.
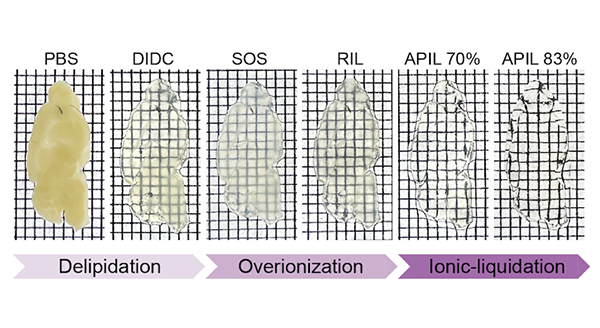
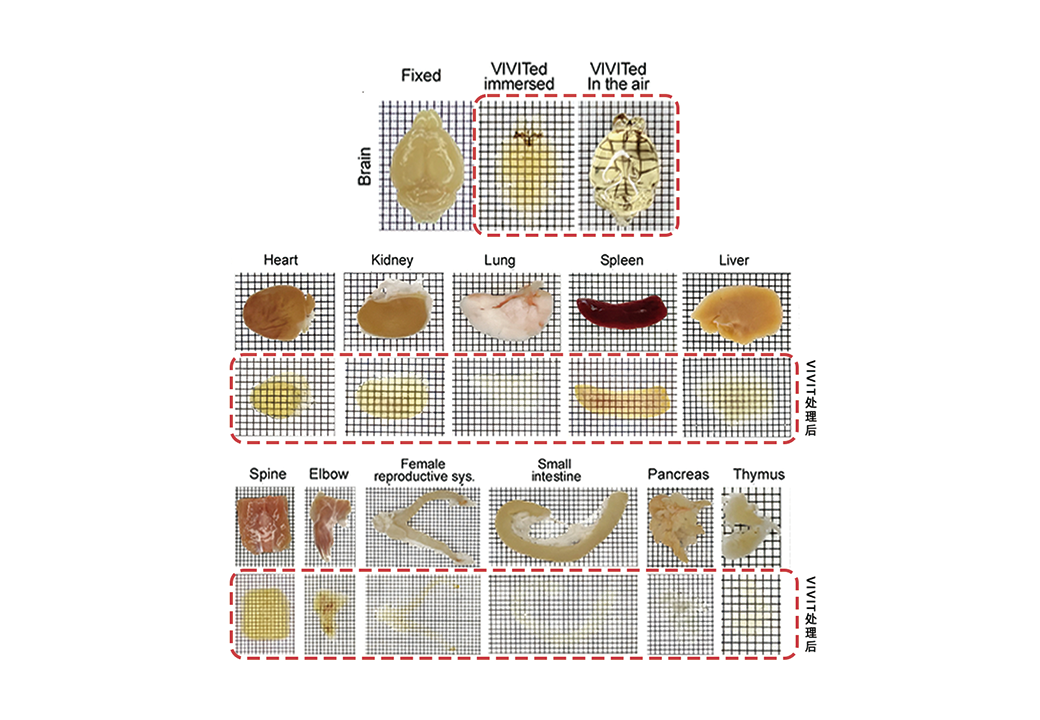
VIVIT offers an innovative solution to this long-standing problem. Unlike conventional tissue-clearing techniques that focus only on transparency, VIVIT also preserves the original structure and fluorescence signals of tissues. With a high-refractive-index ionic liquid developed by the team, VIVIT achieves the transformation of opaque biological tissues into a stable “glassy state” at low temperature for the first time. During the process, tissue expansion or shrinkage is kept within 1%, ensuring minimal distortion. Even in delicate and highly connected samples such as brain tissue, VIVIT maintains the original architecture, allowing subcellular details, including synapses, to be clearly visualized. These capabilities were demonstrated across rodent, non-human primate, and human brain tissues in the study.
VIVIT enables ex vivo tissues to become transparent without deformation.
The core breakthrough of VIVIT goes beyond simply “seeing more clearly.” It also lies in “preserving what matters” and “making data usable.” Experiments showed that after ionic liquid treatment, the signal intensity of many common fluorescent dyes increased by 2- to 30-fold, enabling the detection of faint markers that were previously difficult to observe. At the same time, due to its glassy physical state, VIVIT removes the limitations of cryogenic preservation. Samples can be stored long-term at –80 °C without ice crystal formation, avoiding tearing and mechanical damage. This makes possible truly lossless cryo-sectioning and super-resolution imaging. With its structural fidelity and signal stability, VIVIT provides a solid foundation for acquiring and reconstructing trans-scale 3D tissue data.
-
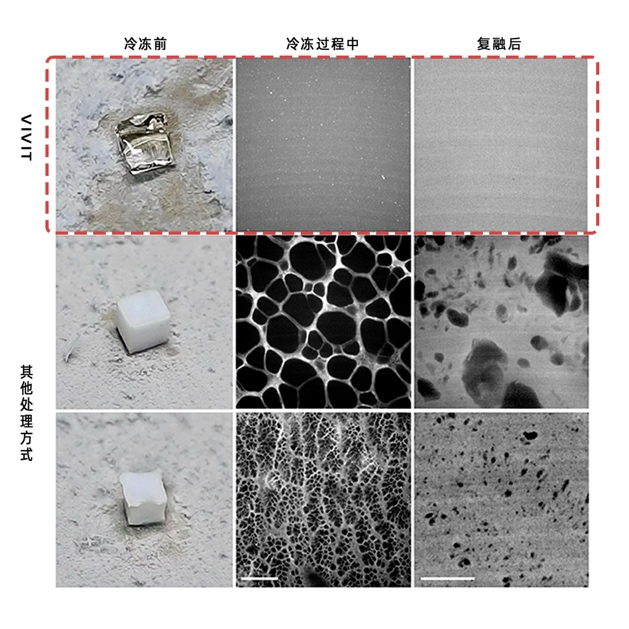 Samples treated with VIVIT show no ice-crystal damage (left)
Samples treated with VIVIT show no ice-crystal damage (left) -
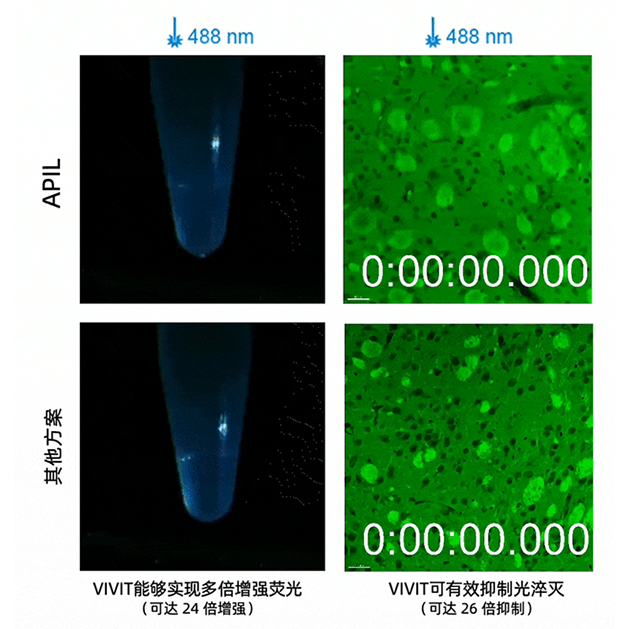 VIVIT enables multi-fold fluorescence enhancement and reduces photobleaching (right)
VIVIT enables multi-fold fluorescence enhancement and reduces photobleaching (right)
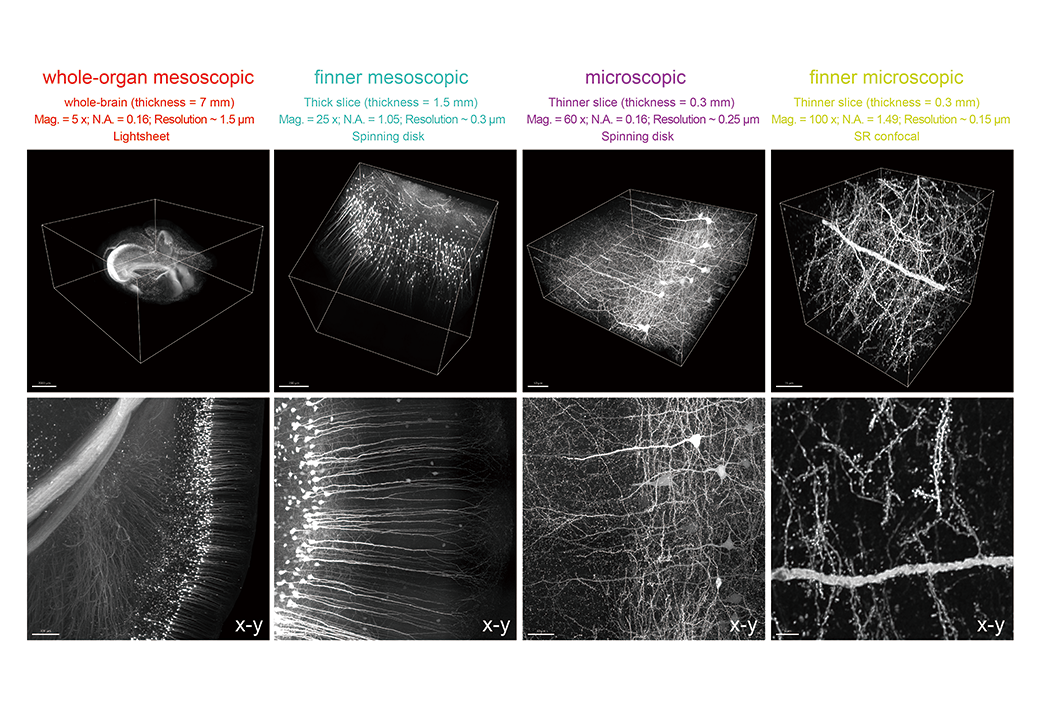
With these unique advantages, Yuan’s team quantitatively linked the sensory modality preferences of neurons in the multimodal thalamus at the synaptic input level (microscale) with their target-region preferences at the whole-brain output level (meso- and macroscale). This marks the first time internationally that accurate input–output mapping has been achieved at the single-neuron level. The breakthrough overcomes a major technical barrier in circuit neuroscience and opens new opportunities for revealing the mechanisms of brain function.
In addition, tissues treated with VIVIT remain compatible with multiple rounds of immunostaining. After each round of staining and imaging, fluorescence signals remain clear and tissue structures stable. This enables researchers to identify multiple molecular targets in the same sample, gaining richer spatial information. At the same time, combined with the team’s self-developed reconstruction algorithm TARRS, VIVIT also supports image stitching from serial tissue sections. This allows the creation of 3D maps that span from subcellular details to entire organs, enabling cross-scale reconstruction of biological structures.
-
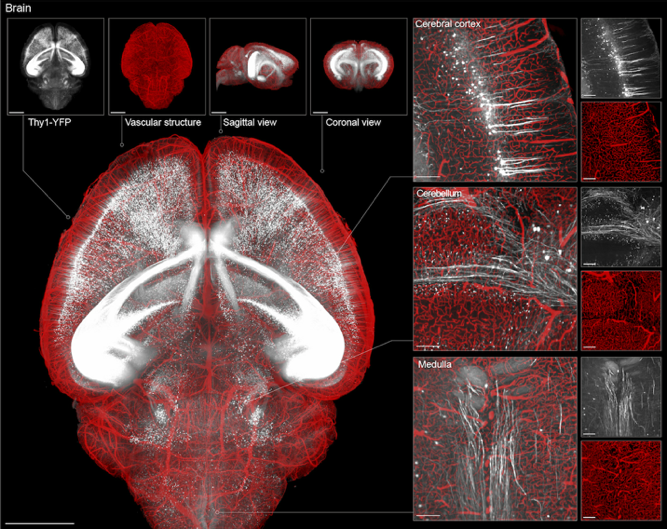
3D reconstruction of brain tissue using VIVIT
-
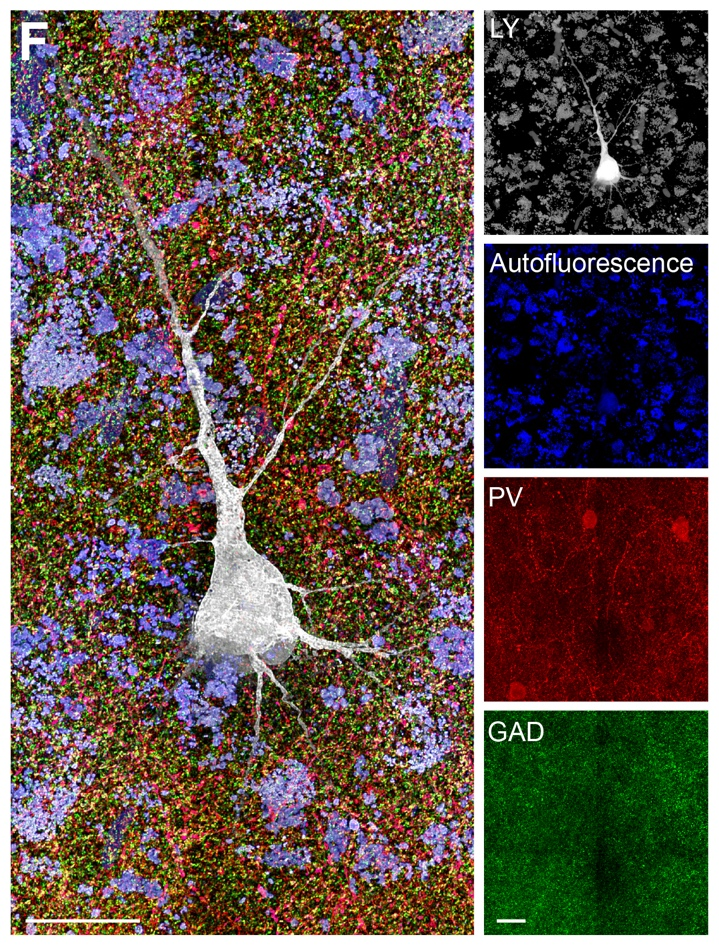
VIVIT reveals micro-connections in the human cerebral cortex
From tissue clearing to multimodal staining and finally to 3D reconstruction, VIVIT presents a complete workflow that links sample preparation with spatial analysis. It offers a systematic solution for acquiring high-resolution, cross-scale spatial data and building accurate models of 3D biological structures.
Full Text:https://doi.org/10.1016/j.cell.2025.07.023
Research Team Introduction
Yuan’s lab at the School of Biomedical Engineering, Tsinghua University, focuses on two closely related yet independent scientific questions: the integration mechanisms of multimodal sensory information and the pathological mechanisms of sensory disorders.
The team conducts original research that spans disciplines, scales, and species, and has published a series of papers in leading international journals. In recent years, they have authored publications as corresponding authors in Cell (2025, DOI link), Neuron (2023), IEEE Transactions on Pattern Analysis and Machine Intelligence (2024), Journal of Neuroscience (2024), and Cerebral Cortex (2019).